Proprietary breakthrough
Rational redesign of HERVs deactivates immunosuppression while maintaining immunogenicity
With just 2 percent of our DNA coding for proteins, the remaining 98 percent comprises elements long considered extraneous, non-functional DNA. Recent insights into the biological impact of these highly repetitive elements have renewed interest in what is now called the “dark genome” or the "regulatory genome" as a source of new disease targets.


HERV genes are methylated and lie dormant when we are healthy and young but are reactivated as we age or develop certain diseases. Upon expression, HERVs can promote dysregulated cell division and suppress the immune system, contributing to the onset and progression of chronic age-related conditions. Despite their known impact on multiple diseases, HERV targeting approaches have remained elusive because the immune system views them as self-antigens.
At HERVolution, we have broken human immune tolerance to HERVs, enabling therapeutic HERV targeting for the first time. We are revolutionizing treatment of complex age-related diseases with proprietary engineering approaches that allow us to make these once-invisible antigens visible to the immune system.
Our rationally redesigned HERV antigens induce potent and durable anti-HERV immune responses and can be expressed using adenoviral, virus-like particle, and mRNA vectors, providing opportunities for expanding our disease targets and dosing range to improve outcomes for the world’s increasingly aging population.
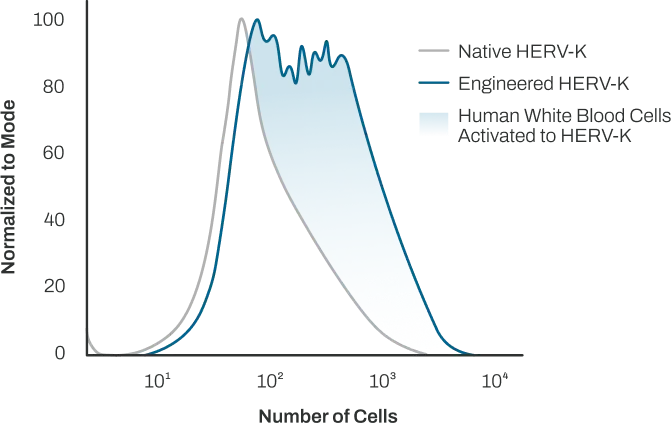
Rational redesign of HERVs deactivates immunosuppression while maintaining immunogenicity
Ready-made HERV-targeted immunotherapies with pipeline-in-a-product potential
Robust data support potential to expand the landscape of targetable HERVs, targeting modalities, and disease indications
Our platform fuels a pipeline of off-the-shelf assets that target HERVs implicated in a range of diseases, with an immediate focus on cancer and metabolic disease and potential to expand into other age-related conditions.

IPT-001, our lead candidate, is a dual adenoviral (Ad) vector immune therapy that offers potential to address a range of diseases as monotherapy or in combination with other agents by delivering a redesigned HERV-K antigen.
IPT-001 utilizes two unique, proprietary Ad-vectors with low pre-existing immunity and excellent antigen expression. These vectors enable simultaneous induction of potent HERV-specific T and B cell responses following a single treatment.
IPT-001 is supported by a robust suite of preclinical data that demonstrate its ability to stimulate powerful immune responses in relevant models. We anticipate initiating clinical evaluation in patients with prostate or pancreatic cancer in 2025.


IPT-002 is an immune therapy that leverages mRNA vectors to deliver a redesigned HERV-K antigen and stimulate strong immune responses when used alone or when combined with another therapy.
The use of mRNA vectors allows for repeat dosing in humans as well as maintenance and amplification of adenovirus-initiated immune responses when administered in a prime-boost treatment strategy with IPT-001.
HERVs have been implicated as contributors to the onset and progression of many well-known diseases of aging. We are advancing rationally redesigned HERVs as a new avenue through which we can address these complex and challenging diseases.
HERVs are an untapped source of tumor-specific antigens that may enable selective cancer targeting and immune engagement of previously cold tumors. HERV proteins are expressed across several cancer types, including pancreatic, prostate, colorectal, breast, and ovarian cancers.
Immunity to HERV-K has been correlated with improved patient responses to existing immune checkpoint inhibitor therapies (eg, anti-PD-1) in lung cancer and renal cell carcinoma.
Our unique immunotherapies contain a viral vector and an RNA vector that stimulate strong antibody and T cell immune responses against HERVs, offering potential for cancer elimination when administered as monotherapy or in combination with approved immunotherapies.
An Endogenous Retrovirus Vaccine Encoding an Envelope with a Mutated Immunosuppressive Domain in Combination with Anti-PD1 Treatment Eradicates Established Tumours in Mice
A Systematic Review of Expression and Immunogenicity of Human Endogenous Retroviral Proteins in Cancer and Discussion of Therapeutic Approaches
The Potential of Adenoviral Vaccine Vectors with Altered Antigen Presentation Capabilities